
- Synthetic Flavours & Fragrances
- Catalyst and Auxiliary
- Agrochemicals
- Organic Chemicals
- Materials
- Basic Inorganic Chemicals
- Pharmaceutical
- Plant Extract
- Reagent
- Dyestuff and Pigment
- Food Additives
- Others

 China (Mainland)
China (Mainland)

Cefatrizine CAS NO.51627-14-6
- FOB Price: USD: 50.00-100.00 /Kilogram Get Latest Price
- Min.Order: 1 Kilogram
- Payment Terms: T/T,MoneyGram,Other
- Available Specifications:
一(1-100)Kilogram一(100-1000)Kilogram
- Product Details
Keywords
- Cefatrizine
- cephatriazine
- s640p
Quick Details
- ProName: Cefatrizine
- CasNo: 51627-14-6
- Molecular Formula: C18H18N6O5S2
- Appearance: White powder
- Application: Ribociclib succinate (LEE011 succinate...
- DeliveryTime: 3-7days
- PackAge: fiber can
- Port: Tianjin Xingang/Qingdao Port
- ProductionCapacity: 100 Metric Ton/Month
- Purity: 98%
- Storage: Seal and store in a cool and dry place
- Transportation: By sea/air/land
- LimitNum: 1 Kilogram
Superiority
1. Product advantages
High purity, all above 98.5%, no impurities after dissolution
We will test each batch to ensure quality
OEM and private brand services designed for free
Various cap colors available
We can also provide MT1 peptide powder
2. Factory advantages
Professional research team
More than 5 doctors in high-tech R&D laboratories
More than 1000 m2 of factory production line to ensure stable supply
More than 1200 factories to manufacture products and control quality
3. Service advantages
24-hour online service
Track package information and update it for customers
Professional sales team
Accept small order service
Redelivery service if detained by customs
Details
Product Introduction:
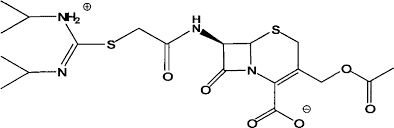
This product is the first generation cephalosporin and is the first in China to be used in clinical practice. The antibacterial spectrum is similar to that of cephalothiophene, with strong effects on Staphylococcus aureus, Streptococcus viridis, and Pneumococcus, and unique antibacterial activity against Enterococcus. It is mainly used for respiratory infections caused by Staphylococcus aureus, Streptococcus pneumoniae, and Streptococcus pneumoniae, as well as infections such as biliary tract infections, urinary tract infections, gynecological infections, sepsis, pneumonia, meningitis, etc.
Pharmacology and Toxicology 1. Pharmacology: This product has antibacterial activity against Gram positive bacteria and some negative bacteria, with a particularly strong effect on Gram positive cocci. The in vitro antibacterial activity test of this product shows that it has strong antibacterial activity against Streptococcus pneumoniae, Streptococcus pyogenes, Staphylococcus aureus (MSSA strain), Staphylococcus epidermidis (MSSE strain), and Streptococcus catarrhalis, with a MIC90 of 0.25 against Streptococcus pneumoniae μ G/ml, 0.5 for Streptococcus pyogenes MIC90 μ G/ml, MIC90 for all three other bacteria is less than 8.0 μ G/ml, also has strong antibacterial activity against Haemophilus influenzae, with a MIC90 of 2.0 μ G/ml. It also shows strong in vitro antibacterial activity against Enterococcus, with a MIC90 of 2.0 μ G/ml. It has good antibacterial effects on Streptococcus viride, Streptococcus hemolyticus, Streptococcus non hemolyticus, Diphtheria, Clostridium perfringens, Tetanus, and Anthrax. The in vitro antibacterial activity of Staphylococcus aureus (MRSA strain) and Staphylococcus epidermidis (MRSE strain) is not as good as that of vancomycin. The mechanism of action of this product is to inhibit the synthesis of sensitive bacterial cell walls, resulting in bactericidal effects. 2. Toxicology: The LD50 of this product injected intravenously into mice is (1.02 ± 0.04) g/kg, while the LD50 injected intraperitoneally is (1.26 ± 0.23) g/kg. In the reproductive toxicity test, the fetal mortality rate of the experimental group mice was significantly higher than that of the control group (p<0.01). Pharmacokinetics: The product is not absorbed orally. After intravenous infusion of 1.0g, the peak blood concentration (Cmax) is (68.93 ± 6.86) mg/L, and the half-life of blood elimination is (t1/2 β) At (1.19 ± 0.12) hours, the area under the blood concentration time curve (AUC) was (94.7 ± 9.8) mg/(L.h), and the cumulative excretion rate of urine medication at 12 hours was 93.1 ± 3.2%. After intramuscular injection of 1.0g, the peak blood concentration (Cmax) was (35.12 ± 4.34) mg/L, the time to peak (tmax) was (0.78 ± 0.08) h, the half-life was (1.38 ± 0.21) h, and the area under the blood concentration time curve (AUC) was (85.3 ± 8.0) mg/(L.h). The cumulative excretion rate of traditional Chinese medicine in urine after 12 hours was 84.2 ± 5.9%. Compared with intravenous drip, its absolute bioavailability is 90.3 ± 6.4%. After injection, the product is widely distributed in the body tissues, with a high content in bile, liver, lungs, and other areas, and does not penetrate the blood cerebrospinal fluid barrier. There is almost no metabolism in the body, mainly excreted from the urine, with over 90% of the dosage excreted in the 12 hour urine. Patients with renal dysfunction have a prolonged serum half-life after intramuscular injection
Hebei Sankai Chemical Technology Co., Ltd. is a new chemical enterprise integrating the research, manufacturing and supply of chemicals. Located in the beautiful "cattle city" Xingtai, Hebei Sankai Chemical Technology Co., Ltd. is mainly engaged in the research of chemical fertilizer. At the same time, we have made great breakthroughs in food, feed additives, cosmetics, dyes and industrial chemicals. Our company spirit of "integrity management, quality control, customer first" belief, at home and abroad enjoy high reputation.






